The “Special Topics” presentation two weeks ago discussed the possibility that signs of life might exist within certain meteorites that have been found to have come from Mars, although it did so partially within the overall context of “are we alone?” Another part of that discussion revolves around how life on Earth – and, hopefully, elsewhere – got started in the first place, and in keeping with the overall focus of “Ice and Stone 2020” includes the roles that the “small bodies” of the solar system played in that process.
An important caveat here is that, when we discuss “life,” we are essentially restricted to “life as we know it,” i.e., the life that we find here on Earth. It is certainly conceivable that life with a completely different structure and nature can and has formed elsewhere, but in the absence of any information about that we have little choice but to go with what we know. The life we do know needs three things to exist and, presumably, to form: energy, water, and organic (i.e., carbon-containing) compounds. Most of the energy would presumably come from sunlight, and in particular – especially in stimulating the formation of life – higher-energy forms of sunlight like ultraviolet (which would have arrived at Earth’s surface in relatively high quantities before the formation of the ozone layer), although geothermal activity, tidal forces, and decay of radioactive substances can also play a part.
Water, not too surprisingly, is one of the most abundant molecules in the universe, and it has been detected in many environments all over the place, including within large molecular clouds from which stars and planets are forming (including within planet-forming disks around young stars), and even within the atmospheres of exoplanets. It has been found all over the solar system, including, obviously, on Earth’s surface and in its atmosphere; there is clear evidence that large amounts of water once flowed on the surface of Mars and there may still be quite a bit of subsurface water in the form of ice. Water ice has been found in the atmospheres of the giant planets, in particular Uranus and Neptune, and many of the moons of these worlds are also made up significantly of water ice. Water geysers have been found erupting off the surface of Saturn’s moon Enceladus, and large worldwide oceans of subsurface liquid water are believed to lie under the surface of Jupiter’s moon Europa and perhaps several other such bodies, including Pluto and – based upon just-published analyses of data from the Dawn mission – the largest main-belt asteroid, Ceres.
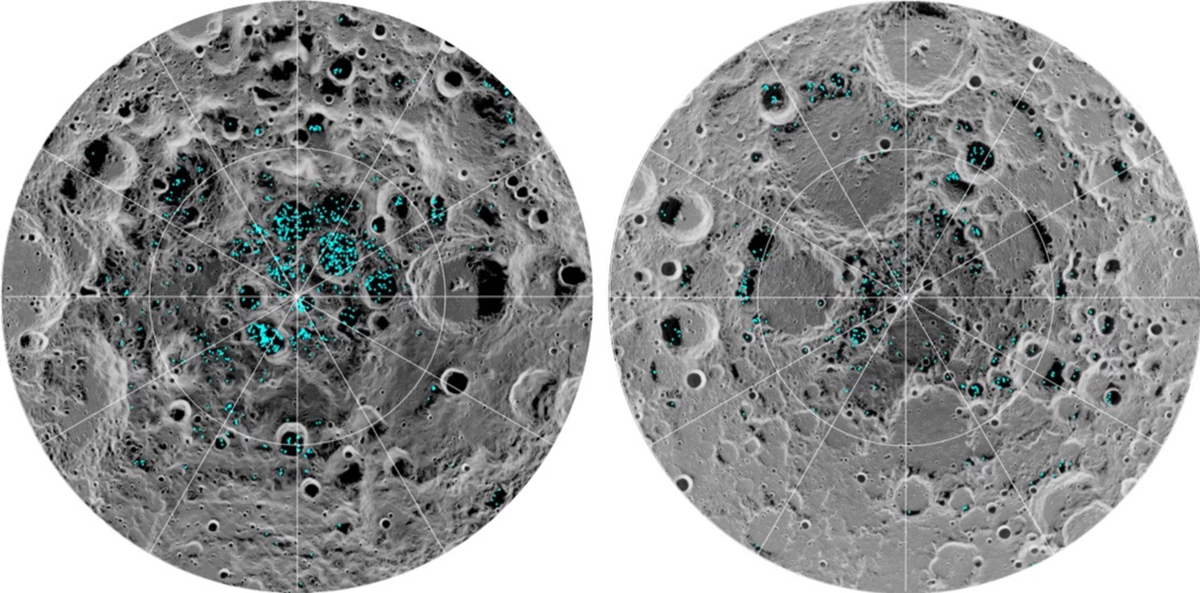
As was covered in the “Special Topics” presentation on Fred Whipple’s comet model, a significant percentage of a comet’s nucleus is made up of water ice, and indeed water has been detected in comets for the past three decades. As comets have impacted the various planets over the history of the solar system they would have delivered water to those bodies. Indeed, this appears to be the most likely explanation for the presence of water ice in what at face value appears to be among the harshest environments for it: the surfaces of Mercury and the moon. Radar observations of Mercury in 1991 suggested the presence of water ice in craters near Mercury’s North Pole in 1991, a result which was confirmed by NASA’s MESSENGER mission in 2012. The presence of water on the moon was first suspected in soil samples returned by the then-Soviet Union’s Luna 24 mission in 1976 and the presence of ice in the moon’s polar regions was strongly suspected in radar experiments conducted by the joint NASA/U.S. Defense Department Clementine mission in 1994. These conclusions were strongly reinforced by NASA’s Lunar Prospector mission in 1998 and the Lunar CRater Observation and Sensing Satellite (LCROSS) mission which deliberately impacted a crater near the moon’s South Pole on October 9, 2009, and completely confirmed by data taken by NASA’s Moon Mineralogy Mapper aboard India’s Chandrayaan-1 mission later that same year and announced in 2018. What appears to be happening on both worlds is that the water in impacting comets is ejected from the impact sites and transported – in the relatively weak surface gravity – to permanently shadowed craters in the polar regions. The temperatures in these environments are very cold, and over the lifetime of the solar system enough such impacts occur so that a non-trivial amount of water can accumulate.
For quite some time it was generally assumed that Earth had received its water primarily as a result of impacts by comets throughout its history. However, an analysis of the water content in several comets, including 1P/Halley, Hyakutake C/1996 B2, and Hale-Bopp C/1995 O1 – all previous “Comets of the Week” – has revealed that the ratio of deuterium to hydrogen atoms within their water is close to twice what it is in Earth’s seawater. Indeed, results obtained by ESA’s Rosetta mission indicate that the deuterium-to-hydrogen ratio in the water of Comet 67P/Churyumov-Gerasimenko is three times that of Earth’s seawater. Meanwhile, an examination of Comet 103P/Hartley 2 – which was encountered by NASA’s EPOXI mission in late 2010 at the same time that the comet was passing close to Earth – by ESA’s infrared-sensitive Herschel Space Observatory indicates that the deuterium-to-hydrogen ratio in that comet is about the same as that in Earth’s seawater. At the very least, then, the overall picture is quite a bit more complex than what had been commonly believed, and it would seem that there were sources for Earth’s water other than comets.

It is conceivable that the so-called “main belt comets,” which are discussed in the forthcoming “Special Topics” presentation on “active asteroids,” could have supplied, at least in part, the early Earth with its water. On the other hand, there are numerous asteroids that also contain significant amounts of water. The carbonaceous chondrite meteorites – which are the subject of their own future “Special Topics” presentation – contain non-trivial amounts of water within their mineral structures, and it would then follow that the parent asteroids of these objects do as well. The large main-belt asteroid (24) Themis – roughly 200 km in diameter – is similar in composition to carbonaceous chondrites, and infrared observations obtained in 2009 indicate that its surface is almost completely covered with water ice. Infrared-sensitive observations have also detected emissions of water vapor from the largest main-belt asteroid, (1) Ceres, and just last year NASA’s OSIRIS-REx mission detected the presence of hydrated (i.e., water-containing) clays on the surface of the near-Earth asteroid (101955) Bennu, and also detected eruptions of surface material – possibly caused by sublimation of water – on several occasions. (This mission is discussed in more detail in a future “Special Topics” presentation.) Whether or not this “asteroidal” water helps clear up the picture as to where Earth’s water originally came from remains to be seen.
Simple organic molecules have been detected within comets for quite some time. More complex organic molecules, including formaldehyde and various long-chained polymers, were detected in and around Comet 1P/Halley by ESA’s Giotto mission when it passed by that object in March 1986. Complex organic molecules like these have since been detected in more recent comets, including Comet Hale-Bopp in 1997, and recently by Rosetta during the course of its examination of Comet 67P/Churyumov-Gerasimenko; overall, Rosetta detected 16 different complex organic compounds, four of which (including acetone) for the very first time within a comet. Organic compounds have also been found in various asteroids, including (24) Themis and (1) Ceres, and also in various meteorites, especially in the carbonaceous chondrites. The presence of organic molecules known as “polycyclic aromatic hydrocarbons,” or PAHs, in the Martian meteorite ALH 84001 was one of the lines of evidence cited as suggesting the presence of fossilized microbial life in that object, as was discussed in the “Special Topics” presentation two weeks ago.

A certain type of organic molecule known as a “tholin” is formed when high-energy radiation, for example, a cosmic ray, interacts with volatile materials such as ices. They do not form naturally on present-day Earth, but have been found in several places in the more distant solar system, such as on the surfaces of icy worlds like Pluto and some of the moons of the outer planets; among other places, tholins are also found in both the atmosphere and on the surface of Saturn’s moon Titan. They are not common further in, although at least one crater (Ernutet) on (1) Ceres contains them, and they have also been detected on (24) Themis and (by Rosetta) on 67P/Churyumov-Gerasimenko. In the far outer solar system, i.e., the Oort Cloud and the Kuiper Belt, tholins should be rather common, and indeed they have been detected on various Kuiper Belt objects and centaurs. The interstellar object 1I/’Oumuamua – the subject of a future “Special Topics” presentation – also appears to contain tholins, as does the recent interstellar Comet 2I/Borisov – a future “Comet of the Week.” Tholins are distinctly reddish in coloration and thus their presence can often be inferred photometrically with different-color filters; among other things, they are believed to be at least partially responsible for the orangish coloration of Titan’s atmosphere and surface that was detected by ESA’s Huygens lander.
Certainly among the more intriguing of the various organic substances that have been detected in astronomical bodies are amino acids, which are utilized by life forms in the performance of basic biological activities. Amino acids have been detected within various carbonaceous chondrites, with over 15 different types of them being found within the Murchison meteorite, one of the most famous and studied of these objects. (Murchison will be discussed more thoroughly in the future “Special Topics” presentation on carbonaceous chondrites.) One of the simpler amino acids, glycine, has been detected in the material samples from Comet 81P/Wild 2 – the Week 1 “Comet of the Week” – that were returned to Earth by NASA’s Stardust mission, and Rosetta also detected glycine in Comet 67P/Churyumov-Gerasimenko.

Finally, two nucleotide bases, uracil and xanthine, have been detected in the Murchison meteorite. Uracil is one of the four bases that make up molecules of ribonucleic acid (RNA).
I should stress that the presence of amino acids and organic compounds like uracil in meteorites like Murchison or in astronomical objects like comets does not mean that life itself is present in these objects. However, their presence, along with the large amounts of water that are present in the solar system, does suggest that the raw materials for life have been around since the early days of the solar system and that the mechanisms for delivering these materials to Earth have long been in action. The same would presumably be true for other potentially life-bearing worlds like Mars, Europa, and Titan, although whether or not life got started on those worlds, and survived like it has on Earth, remains unknown at this time. But in any event, in a very real sense, the study of the “small bodies” of our solar system is an examination of our own origins.
More from Week 34:
This Week in History Comet of the Week Free PDF Download Glossary
Ice and Stone 2020 Home Page